Exploring ISO 9000 - Part 16 Control of Quality Records
A Series of International Standards for Quality Management and Quality Assurance. We begin 2022 with a review of Clause 4.16 Control of Quality Records.
We begin the New Year with a review of Clause 4.16 Control of Quality Records. Four additional ISO Clauses remain and the series will be completed. However, with the introduction of ISO 9001:2000, the series will continue with comparisons and updates to help you and your company better understand QS9000-2000 requirements and conversion from ISO 9000 1994 documentation.
ISO 9001-2000 Second Committee Draft (CD) has been available since February 1999 (see the March 1999 issue of MoldMaking Technology magazine.) The Draft International Standard ISO 9001-2000 will be available for purchase January 15, 2000.
Introduction
ANSI/ISO/ASQ Q9000-2-1997 states: "The supplier's quality records should give evidence that quality system elements falling within the requirements of Q9001-2 or 9003 have been implemented. If the results have not proved satisfactory, quality records should indicate what has been done to correct the situation."
The approach for control of quality records is to identify the records required in each of your procedures to support or provide objective evidence that the procedure has been followed. It is suggested that you develop one procedure to comply with Clause 4.16.
The following list of clauses is provided to identify Clause 4.16 quality record requirements.
4.1.3 Management Review
4.2.3 Quality Planning
4.3 Contract Review
4.4.5 Design Output
4.4.6 Design Review
4.4.7 Design Verification
4.6.2 Acceptable Subcontractors
4.7 Control of Customer Supplied Product
4.8 Product Traceability
4.9 Process Control
4.10.2.3 Urgent Production Release
4.10.4 Final Inspection & Testing
4.10.5 Inspection and Test Records
4.11.1 Control of Inspection, M & T Equipment
4.11.2.e Control Procedure
4.13.2 Review and Disposition Nonconforming Product
4.14.1 Corrective/Preventive Action
4.14.2 b Results of Nonconforming Work
4.16 Subcontractor Quality Records
4.17 Internal Quality Audits Training
One easy method of meeting quality records requirements is to develop a record matrix as part of your Clause 4.16 procedure. The matrix headers (see Table I) provide clear guidance for each responsible party within your organization.
Clause 4.16 Control of Quality Records
"The supplier shall establish and maintain documented procedures for identification, collection, indexing, access, filing, storage, maintenance and disposition of quality records."
There are eight specific procedural requirements that will be individually addressed:
1. Identification of Quality Records
Action Item
Refer to Table I. The matrix identifies each of the quality records by department. Identification is also accomplished in each of your procedures addressing the 20 elements of the standard. Within each procedure, identify forms, reports, lists, charts or any other required quality record.
2. Collection of Quality Records
Action Item
When you identify a quality record in a procedure be sure to include what happens to the record after a specification is completed. This direction will explain whom the record passes on to, is submitted to or where the records are collected. For example: the in-process inspector initials block "x" on the traveler indicating inspection passed. The traveler remains with the part until final inspection.
If you use a computer network, data collection is a simple step and completed for you using system files and directories. Records are easily collected for analysis or other use.
3. Indexing Quality Records
Action Item
This requirement means to ensure that you have all records identified (i.e., that no records are missing). There are various methods of indexing, such as use of checklists, sequential numbering systems, color coding, by project, by part number, by job number or by customer. In practice, indexing requirements are met by continuing existing filing systems with slight modification, if any. Be aware that you could be using several indexing systems between different departments within your organization.
4. Access to Quality Records
Action Item
The access requirement addresses the ability to retrieve and security of quality records. This section is out of order. It should have followed the file requirement. You need to provide access to records for those individuals needing such access and individuals approved for such access. Security may be an issue within your company for specific records like training, usually located in the Human Resource employee file. Describe who has access and who is prohibited from access to specific records in your procedure. If your system is online, access may be restricted by password. Additional security might require locking of file rooms, cabinets and use of sign-in and out logs.
5. Filing Quality Records
Action Item
Indexing and filing are connected requirements. Your record's matrix identifies storage locations so anyone authorized can retrieve a record as needed. The method of filing used will be identified by the department responsible for the quality record(s). If you looked at the quality record's matrix for Management Review, you may see the location identified as Management Representative Office. Visiting the MR Office, you might find the filing system is to use a three-ring binder containing dated records for each meeting. Visiting the Purchasing Department looking for supplier records may result in finding an alpha file by supplier.
6. Storage of Quality Records
Action Item
As noted in requirement five, storage location is identified by the matrix. Besides storage location, you must consider environmental conditions and loss prevention actions. Storage addresses office storage for current records and records archived for future reference. Environmental storage conditions include questions about the area. Is it dry, vented, heated, covered, lighted, accessible? Are records stored on the floor, racks, shelves, bins, etc.? Is pest control an issue?
For loss prevention requirements address the possibilities of fire or theft. Does the area have a fire sprinkler system or fire alarm system? If yes, how often is it tested and who maintains it? Is the area secured by a lock prohibiting easy entry? How frequently is the area policed or checked for security? Identify who has access by key, title or other means. If your system is computerized, document back-up procedures and frequency. List storage areas for back-up files and who has access to those files to deal with viruses and corrupted files.
7. Maintenance of Quality Records
Action Item
Maintenance includes filing, collection and storage issues. Your filing system provides a means for records to be readily retrievable. Maintaining the files means keeping the files up-to-date, placed in the proper location and not allowing alterations to completed records. For example: asking the Human Resources department for a specific training record. The human resources person checks the employee file and does not find the record. The person checks the pile of unfilled records and after 20 minutes has not found the requested record. This situation indicates inadequate maintenance of records. The file was not in the proper location. Another common maintenance issue is keeping shop floor records clean. A simple solution is to use a clear-view protective cover.
8. Disposition of Quality Records
Action Item
Disposition refers to a method of disposal of quality records. Your records retention matrix or procedure identifies the length of time records are to be maintained. This is usually tied to the length of a contract, life of a product, legal requirements or audit cycle. Next, identify what your disposal schedule or frequency is and define how you dispose of specific records. Consider the security issues. Do you shred, burn or manually tear? Don't use the dumpster or even local trash hauler to dispose of proprietary records, customer records, drawings/prints or any record of value to a competitor unless they are destroyed.
Continuing With Clause 4.16 Requirements
"Quality records shall be maintained to demonstrate conformance to specified requirements and the effective operation of the quality system."
Action Item
Q9000-2-1997, Item 4.16 states:
"Quality records should give evidence directly or indirectly as to whether or not the product meets specified requirements."
Evidence is identified by your procedures including Management Review, Process Control, Inspection Requirements, Quality Audits and Corrective and Preventive Action and Customer Complaints. Conformance to requirements and operation effectiveness is validated by the analysis of customer complaints, nonconforming product, results of internal audits and third party audits, and yearly management reviews at a minimum.
Clause 4.16 Requirements
"Pertinent quality records from the subcontractors shall be an element of these data."
Action Item
Treat subcontractor records the same as all other quality records. Specify records requirements on each subcontractor Purchase Order to eliminate any confusion. If you conduct subcontractor audits, gain agreement about what records may leave the facility as part of audit documentation before conducting the audit. Be sure to include subcontractor records in your record retention matrix.
Clause 4.16 Requirements
"Where agreed contractually, quality records shall be made available for evaluation by the customer or the customer's representative for an agreed period."
Action Item
This requirement only applies when contractually required. Be careful with a contract requirement causing you to change your record practices for one customer. Try to help the customer understand your system and how you can provide the required records in the existing format. Show the customer how your records are filed, retrieved and maintained to meet their needs. If the customer requires you to do something different from your existing practices be sure to follow your document change or update process. Use a quality bulletin or other temporary communication method to all concerned.
Summary - A Real Life Quality Records Story
Quality Systems Update, a monthly publication from The McGraw Hill Companies, recently reported about a federal judge in Arkansas who ordered a farm machinery manufacturer to turn over the ISO manual and materials in connection with a product liability lawsuit. The company must turn over its quality system documents to attorneys seeking damages over alleged design flaws. Attorneys stated that the manual was significant because they wanted to find out if the manufacturer complied with its own requirements.
Looking forward, the manufacturer will probably produce quality records supporting its position that it in fact did follow documented procedures proving it is not liable. This is the first case of its type. The message is clear: Be sure your quality record system is efficient and effectively maintained to support the requirements of your Quality System.
Related Content
How to Analyze and Optimize Cutting Conditions to Reduce Cycle Time
Plastic injection mold design and manufacturing company puts NC program optimization software module to the test. The results were surprising.
Read MoreMaintaining a Wire EDM Machine
To achieve the ultimate capability and level of productivity from your wire EDM on a consistent, repeatable and reliable basis, regular maintenance is a required task.
Read MoreIt Starts With the Part: A Plastic Part Checklist Ensures Good Mold Design
All successful mold build projects start with examining the part to be molded to ensure it is moldable and will meet the customers' production objectives.
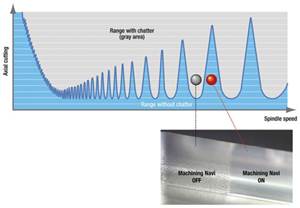
Read MoreHow to Eliminate Chatter
Here are techniques commonly used to combat chatter and guidelines to establish a foundation for optimizing the moldmaking process.
Read MoreRead Next
ISO 9000 Model 1 - Quality Issues as a Part of Good Business
Solutions for managing the plastics supply chain improving OEMs' total cost of manufacturing.
Read More