Tool Steel and Heat Treatment, Part 1
An introduction to heat treatment for the moldmaker.
|
Metals have been used by mankind since man stopped using flint as a tool for scraping hides and killing animals for meat. Man knew about iron, but could not raise a melting temperature high enough to extract the iron from the ore. So easier metals were used such as copper. Man discovered how to alloy copper to make brasses and bronzes for many different applications, such as sword making, armor, shields, spears and many other applications. It was approximately 4,500 years ago that the Chinese discovered how to raise the temperature of iron to such a temperature that it would melt. It was through a simple reciprocating bellows system that kept a continuous stream of air to the fire and made it hot enough to melt the iron. Iron was then used for tools for scraping, raking, hoeing, tilling, and so on. So toolmaking really started to use iron approximately 4,500 years ago. Carbon was introduced into the iron when the iron was being forged. The carbon source was from the forge fire when wood/charcoal was being burned. There is evidence of martensite being created by the ancient Chinese and also in India with Wootz iron. The ancients were not able to identify the martensite that was formed, only that they knew if you heated the iron and cooled it down, it became hard. It was with the advent and use of the metallurgical microscope that martensite (and other structures) were able to be identified and named. There is reference in Homer’s Odyssey to the use of iron and the iron being tempered. It was around the mid-nineteenth century that the effects of adding other metals into the molten iron was experimented with., and then the effects of alloying the steel were investigated. Alloying of steel (iron plus carbon) using different alloying elements really came into its own at the turn of the 20th century.
What Is Heat Treatment?So now that you’ve had a brief introduction into the history of tool steels, let’s get to the basics of heat treatment. Heat treatment of metal is a method of manipulating the metal (in terms of its physical properties) to achieve the operational condition of the metal—both for machining and then for operation. Most metals can be made to be soft or hard, or tough or wear-resistant or both tough and wear-resistant. They also can be made to reduce the possibility of corrosion or they can be made to be soft. This is all done with the application of heat to the metal. There are many definitions of metals:
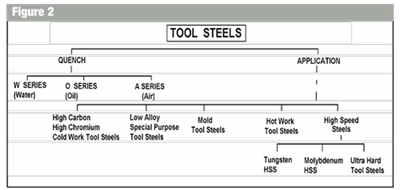
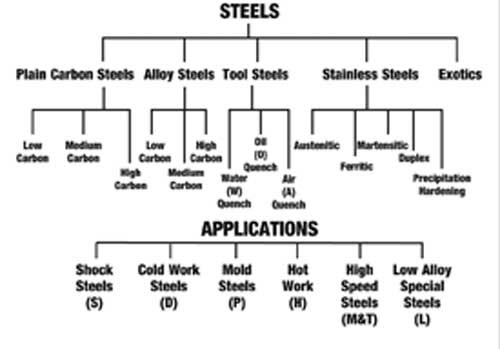
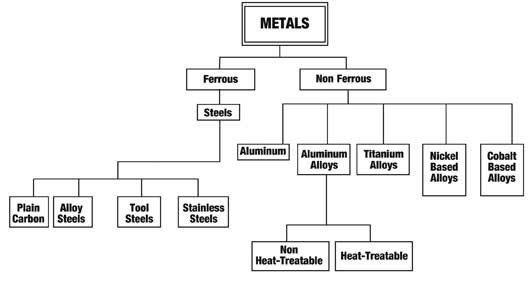
In our case metals are usually alloyed with other elements to change the physical condition of the metal, such as, iron with carbon to make simple steels and copper with zinc to make simple brass. There are many combinations of mixtures of metal elements to give equally, many different characteristics. The metals groups can be divided into two distinct categories (see Figure 1). It can be seen from the tree that we have focused only on steels (ferrous) and other metals (non ferrous). In this series we will be focusing on the ferrous group of metals (steels). What Is Steel? The tree is a very simplified method of both identifying and categorizing steels. A similar tree is done for the non-ferrous metals that illustrates how the non-ferrous metal groups are distinguished from each other. The applications section of Figure 2 is an extension of the tool steel category, which shows the second group of tool steels. Tool steels by quench method and tool steels by application methods are shown in the schematic tree.
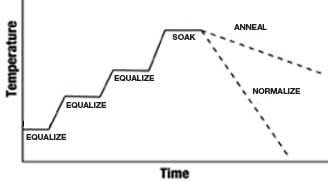
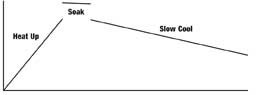
Simple Heat Treatment MetallurgyThe heat treatment of any steel simply means that you will apply heat to the steel to raise it to a required temperature and then cool it down in an appropriate manner. Figure 3 demonstrates the heat treatment process. The schematic illustrates a simple process of heat up, soak at temperature and cool down, which represents the basic schematic for every type of heat treatment that one can think of—including surface treatment processes. Heat treatment is used to make the steel soft for machining and manipulation, and then into the final metallurgy necessary for the steel to function in its particular environment. The next article will continue the thermal process technology definition and also will continue with tool steel classification.
|
|
Editor’s note: You can read the next part in the series by clicking here.
Related Content
Moldmakers Deserve a Total Production Solution
Stability, spindle speed and software are essential consideration for your moldmaking machine tool.
Read MoreAdvances in P20 Steel Potentially Eliminates Need for Stress Relieving After Rough Cutting
Omega Tool Corp. compares conventional, new P20 grades side by side in production fascia tools, finds no downside.
Read MoreHow to Lower Cycle Times With the Right Tool Steel
Combining excellent mechanical properties, high wear resistance and high thermal conductivity in a specialty tool steel yields cycle time reduction.
Read MoreSelf-Venting Mold Steel for Defect Prevention
High-tonnage pressed and sintered, porous metal Vortex removes the difficulties of trapped gas in the mold cavity through a system of interconnected pores.
Read MoreRead Next
Identifying Common Causes of Failure in Ejector Sleeves
Criteria that can be used in making ejector sleeves, which will minimize or eliminate failures as well as improve the performance and function of ejector sleeves and minimize maintenance.
Read MoreTool Steel and Heat Treatment, Part 2
Tool steel, its classification, heat treatment and how to consider manufacturing methods in relation to heat treatment and the metallurgy of heat treatment.
Read More



_300x250 4.png;maxWidth=300;quality=90)











.jpg;maxWidth=300;quality=90)